It’s easy to overlook. Tucked away on the back of a supplement bottle—often beneath dosage instructions or a list of obscure-sounding ingredients—is a modest, three-letter emblem: NSF. It makes no extravagant claims, offers no glowing endorsements, and rarely features in the marketing language plastered across the front of the label. And yet, for those who understand its implications, that quiet mark is one of the most meaningful forms of assurance available to consumers in an industry rife with ambiguity.
The supplement industry is a paradoxical space: vast, fast-growing, and largely self-regulated. Unlike pharmaceuticals, supplements are not required to demonstrate efficacy or undergo rigorous pre-market testing before being sold. In this environment, the burden of verification often falls on the consumer—or, if they’re fortunate, on third-party certifiers like NSF International. This nonprofit organization does not traffic in hype. Instead, it traffics in verification.
A History of Oversight, Not Overstatement
NSF International traces its origins to 1944, when it was founded as the National Sanitation Foundation. Its initial mandate focused on food safety and public health—areas in which scientific rigor and consistency were paramount. Over time, the organization expanded its reach, eventually becoming a recognized standard-bearer across industries ranging from municipal water systems to dietary supplements.
Today, NSF operates globally as an independent certifying body. Its role within the supplement space is both precise and essential: to confirm that what appears on the label corresponds to what is actually in the bottle. This is not a superficial exercise. It involves chemical analysis, contaminant screening, manufacturing audits, and a degree of scrutiny that few brands voluntarily subject themselves to unless transparency is truly a priority.
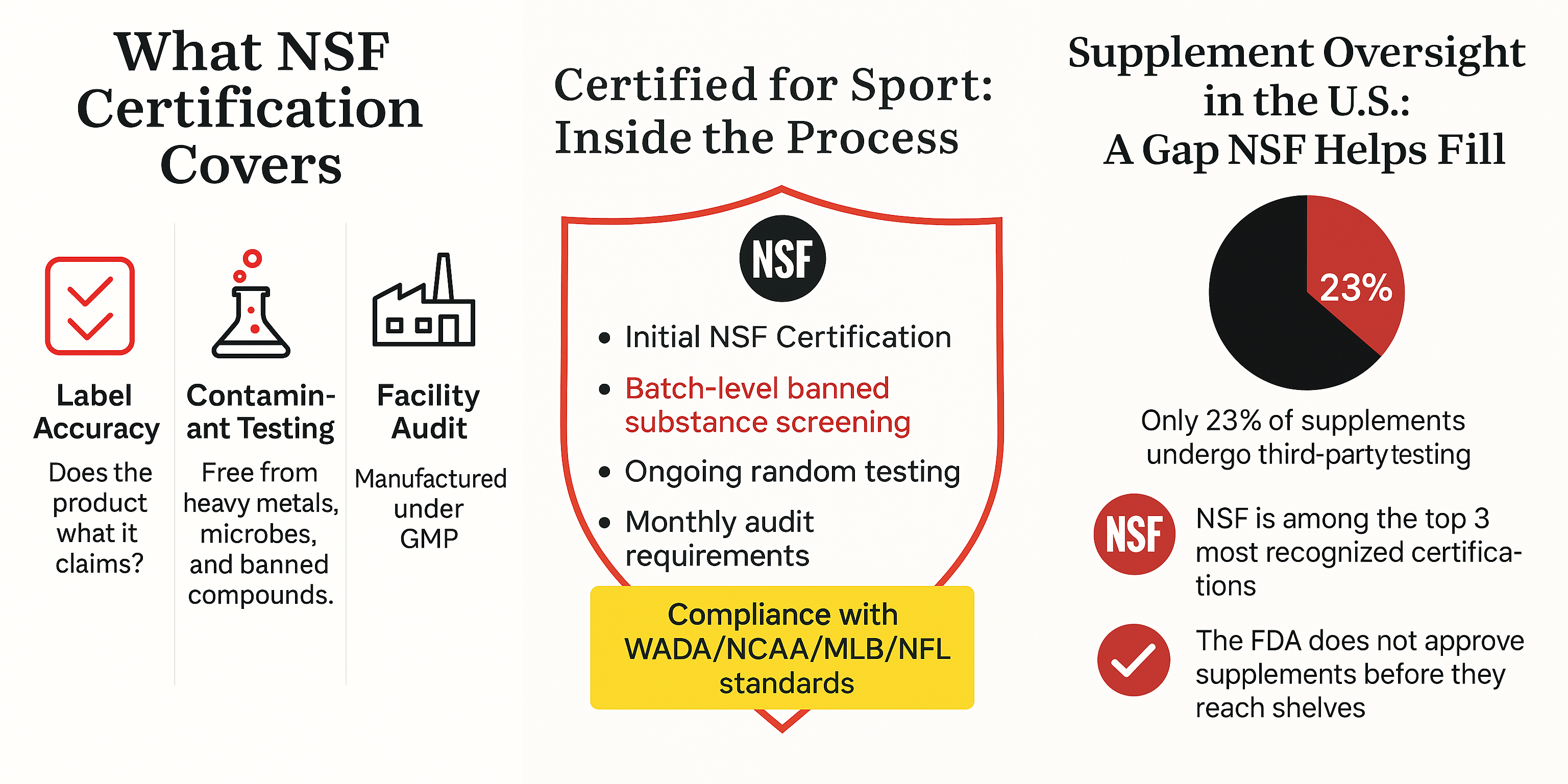
What Certification Conveys—And What It Does Not
NSF certification is best understood as a statement of integrity. It indicates that the product has been tested for label accuracy, screened for contaminants such as heavy metals or pathogens, and manufactured in accordance with FDA Good Manufacturing Practices (GMP). In other words, it verifies the absence of misleading claims, harmful adulterants, or unapproved additives.
Importantly, NSF certification does not speak to whether a supplement is effective. It does not weigh in on clinical outcomes, optimal dosages, or therapeutic value. That work belongs to researchers, clinicians, and regulatory scientists. What NSF offers instead is foundational: a verified starting point from which further inquiry—and trust—can begin.
Certified for Sport: Assurance at a Higher Standard
Among NSF’s offerings is a specialized designation known as Certified for Sport, which caters to elite athletes and professional sports organizations. This program extends beyond basic certification by screening for more than 280 substances banned by the World Anti-Doping Agency (WADA), the NCAA, and major North American sports leagues.
For athletes, the presence of a banned substance—even inadvertently consumed—can carry significant consequences, including suspensions and reputational damage. Certified for Sport provides an added layer of protection by ensuring products are not only clean but compliant with stringent athletic standards. While designed for high-performance contexts, this certification is increasingly sought after by everyday consumers who demand the same degree of assurance in what they ingest.
Why Third-Party Certification Still Matters
In a marketplace characterized by low barriers to entry and limited regulatory oversight, NSF certification offers something that marketing cannot: independent validation. Supplements are frequently manufactured by third parties, relabeled by private brands, and sold under a variety of health claims. Without mechanisms for accountability, consumers are left to navigate a complex and inconsistent landscape with limited tools for discernment.
NSF does not eliminate that complexity, but it mitigates risk. It allows consumers to focus their attention not on whether a product is safe or accurately labeled, but on whether it suits their needs. That is a meaningful shift, particularly for those who rely on supplements for ongoing health management, performance optimization, or condition-specific support.
The Bottom Line: Trust Is Earned, Not Marketed
Not every high-quality product bears NSF certification. Some manufacturers may choose other respected programs, such as USP or Informed Choice, while others forgo certification entirely due to cost or complexity. But when the NSF mark is present, it represents a level of diligence that few brands pursue without reason. It signals a commitment to transparency, quality, and the kind of quiet excellence that doesn’t need to shout to be heard.
In an industry often dominated by bold claims and weak evidence, NSF certification is a rare signal of restraint—and a reminder that sometimes, the most credible assurance is the one that simply says: we checked.
